-
自动化测试设备
这些系统以业界领先的精度和成本效率测试半导体性能和质量。为半导体设计评估、批量生产和产量提高提供强有力的支持。

-
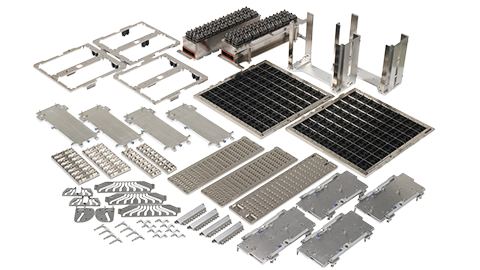
测试系统相关组件
这些产品包括与测试系统配套使用的测试外围设备,如连接半导体器件与测试系统的接口,以及将半导体传送到测试系统的处理装置。系统级测试设备和软件也属于这一类。
-
CD-SEM(关键尺寸扫描电子显微镜)
我们的扫描电子显微镜产品采用扫描电子显微镜技术,以高精度和高稳定性测量和审查微小的表面结构,如晶圆上的光掩膜蚀刻和电路。

-
Advantest Cloud Solutions™
我们的数据基础架构从每个半导体工艺中收集数据,并使用人工智能和机器学习对其进行分析,从而创建实时优化的解决方案,使整个半导体价值链的生产率得到提高。

-
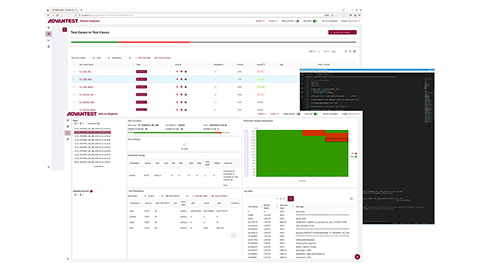
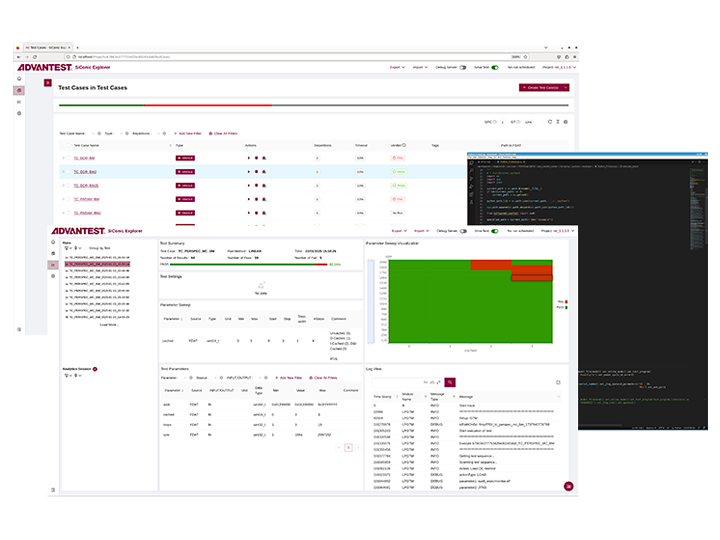
Advantest SiConic™
Advantest的统一环境为自动化硅验证提供了可扩展的生态系统与解决方案。